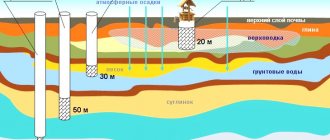
A well in a country house, garden plot or in a vegetable garden allows you to successfully solve the problem of providing technical and drinking water in the absence of a centralized water supply or a well. However, many owners are forced to struggle with the problem of how to clean iron from water from a well with their own hands.
If a yellow-brown or orange liquid flows from the tap, everything is clear without analysis: the water needs iron removal
Ferrous waters are a fairly common occurrence. Iron impurities can be removed from water using special reagents, filters, etc. Most often, such purification requires substantial financial investments. However, there are effective and yet inexpensive ways to purify water. Let's take a closer look at the dangers of exceeding the norm for iron content in water and how it can be reduced at the lowest cost.
Characteristic signs
What to do in cases where it is not possible to quickly conduct laboratory analysis? You can determine that there is a lot of iron in water from a well by organoleptic signs:
- Metallic taste.
- The appearance of red stains on plumbing fixtures that are difficult to get rid of.
- The red, gelatinous sediment begins to smell unpleasant when it comes into contact with air.
- A rust-colored sediment when heated.
- Changing the shade of laundry after washing.
What filters will help reduce the amount of iron in water?
The following will help reduce the concentration of metal in water:
- main filters - their operating principle is reminiscent of water purification in a jug. Water is forced through a special cartridge;
- titanium filters - remove two forms of iron. Effective in cold and hot water. Compact. Cartridges do not need replacement, just washing is enough. The products have no restrictions on the period of operation;
- magnetic - overhead type devices. Special magnets are mounted on the surface of the pipe. The principle of operation of a magnetic field works. They have many disadvantages, so they are used quite rarely;
- polyphosphate - in addition to purification, they make the water softer. They are a flask made of plastic, inside of which there is a filler. The reagent is sodium polyphosphate particles.
Samples
The research is carried out by mobile laboratories that go to the collection site under concluded contracts, and by the SES.
Important! Only accredited laboratories that have received documented permission can carry out analyzes.
It is important for the consumer to know how to correctly collect samples for analysis of iron in water from a well: DETAILS HERE
Norm
After the analysis, a test report is issued.
The permissible norm for Russia is 0.3 mg/l.
Consequences of underperformance or overperformance.
Excess, as well as deficiency of a chemical element in the body, negatively affects the health and well-being of a person.
Elevated metal levels cause:
- Deposits of the element in tissues and internal organs.
- Headache, fatigue, dizziness.
- Change in skin color.
- Problems with the gastrointestinal tract - nausea, vomiting, intestinal ulcers.
- Liver and kidney failure.
- Diseases of the heart and blood vessels.
- Risk of malignant tumors.
- Anemia.
A reduced content of a chemical element provokes:
- A decrease in the concentration of hemoglobin, which is involved in the transport of oxygen to organs, tissues, and the brain.
- Decreased muscle tone.
- Mental disorder.
- Decreased immunity.
- Increase in body weight.
Important! The main reason for the increase in iron concentration in the human body is its excess intake from drinking water.
How is water analyzed?
Having drilled a well on the site, you cannot immediately use the water. It is important to carry out appropriate chemical analysis to ensure proper water quality. This is a matter of the safety of the liquid for health, and not a whim of marketers.
How to analyze water
Thus, the analysis is carried out by certain organizations that have the appropriate authority, license and equipment. You should not be fooled by the low cost of services - it is better to choose a proven laboratory. If you work with intermediaries, you can get false test results.
Whoever will carry out the analysis must take water samples. When the well is drilled, you can invite a specialist. It is advisable to call laboratory technicians a couple of weeks after the construction of the well - then there will be less various contaminants and other third-party substances in the water that entered the reservoir during the construction of the well.
How to recognize the presence of iron in water
Water is collected in clean laboratory containers to avoid errors. If samples are taken independently, then it is important to follow simple rules: take water with clean hands into a container that does not smell anything and is well washed. Moreover, before taking the liquid, you should rinse the container with this same liquid a couple of times. It is better to drive water through the well for 5 minutes before taking it. You need to pour water into the container in a thin stream along the wall of the container to the very top, so that there is no room for air to accumulate.
Important! Be sure to write on the container the time and date of water collection, as well as the location of sampling. Water must be delivered to the laboratory within 2 hours after collection. If this is not possible, then the time can be increased to 10 hours, but the water should be in the refrigerator.
Water analysis result
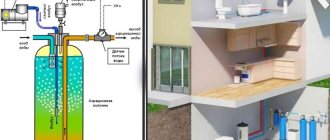
Iron removal plants for cottages and homes
To accelerate the chemical oxidation reaction, use:
- Aeration.
- Ozonation.
- Ion exchange.
- Chlorination.
- Reverse osmosis.
- Use of hypochlorite.
- Introduction of reagents and catalysts.
Aeration
Injected oxygen oxidizes divalent iron, removing carbon dioxide, which also accelerates the oxidation process.
The following methods are used for this:
- Flowing with spray installations;
- Spraying – smothering;
- Air injection by compressors.
The above methods are effectively used in the presence of iron up to 10 mg/dm3. In cases where the concentration is exceeded, to maintain the intensity of the process, preliminary water treatment is carried out using the aeration method with the introduction of reagents (chlorine, sodium hypochlorite, potassium permanganate).
Ozonation
The method is based on the structure of the ozone molecule. The element is unstable and easily gives up an extra oxygen atom, which is an active oxidizing agent. By combining with molecules of other substances, it actively oxidizes and destroys them.
In addition to iron, ozonation helps purify liquids from insoluble magnesium and calcium compounds that can be removed by mechanical filtration.
It also disinfects, discolors, and removes foreign odors and tastes. During ozonation, many bacteria die and toxic impurities are removed.
Ion exchange
You can remove iron from water using an ion exchange resin.
In recent years, natural components have been replaced with synthetic resins that are highly effective. The main task of filtration using the ion exchange method is to get rid of other divalent metals: calcium and magnesium.
In laboratory conditions, this method will remove high concentrations of metal, but on an industrial scale it is difficult to use the method. The presence of oxygen in the liquid passing through the ion exchanger causes precipitation and rapid clogging of the sorbent. The process has to be stopped to wash the resin.
Ferric iron reduces the effective removal of calcium and manganese. The resin quickly becomes overgrown with an organic film.
The ion exchange method is used when additional water purification is necessary.
Chlorination
Chlorine is an oxidizing agent that accelerates the process of converting an element from a divalent to a trivalent form. Chlorination solves the problem of disinfection, removal of hydrogen sulfide and manganese, and organic substances.
Liquid chlorine is highly toxic; delivery and handling of it requires compliance with strict safety measures.
Hypochlorite
It is served by dosing pumps. At the same time, the necessary proportions for different degrees of contamination are observed.
Benefits of sodium hypochlorite:
- The solution of the substance does not form suspensions and does not require settling.
- The use of hypochlorite does not increase water hardness compared to bleach solutions.
- The chemical is obtained at the filtration site by electrolysis of table salt - a substance that is safe for transportation.
- The drug has bactericidal properties - the process of cleaning from metal is combined with disinfection.
The calculation of the dosing installation is made on the basis of data obtained from a chemical laboratory analysis of the composition of the liquid. In addition to the iron content, the presence of heavy metals and hydrogen sulfide is taken into account.
Types of iron impurities
Like any element, Fe can exist in the form of various compounds with other components, and the number of bonds formed with atoms varies. The following types are distinguished:
- Divalent oxide. When it enters a water mass it becomes trivalent, and it acquires the color of rust.
- Bivalent iron, the removal of which from water is necessary if the molecule consists of two atoms. The presence of this component is difficult to see without equipment, since it is soluble and does not change the color of the solution.
- Ferric iron, which usually produces a precipitate.
- Organic suspensions.
Most often, iron-containing fractions of different types are contained, so when choosing a filter, you need to take care to remove all varieties.
Traditional methods of deferrization
Folk, or old-fashioned, purification methods are used in cases where obtaining clean water is required from time to time and the purchase of expensive equipment is impractical.
Advocacy
This is a simple, least expensive method of deferrization.
To implement the home method, you will need a reservoir equal to the daily fluid consumption. Use a container made of neutral materials - food-grade plastic, stainless metal.
The manufacturing process is simple; cheap components are used in the design.
To prevent freezing in winter, the container is placed in a room with a positive temperature.
A shut-off valve is installed at the inlet to prevent overflow. The oxidation process is accelerated by a compressor. Water is supplied to the container through a food hose with a spray nozzle at the end of the tube.
There are two holes at the bottom of the tank:
- The first, at the bottom level, will be used to drain dirty water with flakes.
- The second hole is made at a level of 20-30 cm above the bottom, through which the clarified liquid is taken.
Important! Clean water is taken no earlier than 10-15 minutes after the last air supply. Otherwise, mixed dregs will enter the house. To improve cleaning, magnets are installed that attract iron residues.
Advantages of the method:
- Simplicity and the ability to independently manufacture a sump.
- A water reserve is created in case of a power outage.
- Hydrogen sulfide present in artesian wells is removed from it.
Flaws:
- Incomplete removal of iron.
- Labor-intensive maintenance. It is necessary to regularly drain the sediment and periodically wash the walls of the container from sediment. The frequency depends on the degree of water contamination.
- It is necessary to monitor the liquid level in the reservoir.
Aeration
This method and the principle of its effect on water were described above. The method can be used at home. For this purpose, a special installation is made. The operating principle can be understood from the figure.
Boiling, freezing
The methods are used to obtain a small amount of clean water.
Iron precipitates after 10 minutes of boiling.
Freezing allows you to combat salt impurities. The water is placed in the freezer. Pure water molecules freeze first; salts turn into ice at lower temperatures. After freezing half the volume of liquid, the remainder is drained. Thawed ice is pure water without impurities.
Purifying water from iron requires a careful and responsible approach. Self-purification is a method applicable to obtaining small volumes of liquid for one-time use. The best option would be to contact specialized organizations to purchase and properly install a filter system. This will allow you to avoid mistakes when choosing equipment, installing it, and will allow you to obtain a guarantee.
Drawing conclusions
The effect of filtration depends on the degree of presence of harmful impurities. If it contains ferrous substances of organic origin, the hardness is increased, multi-stage purification is needed. This will include a coarse filtration filter, then aeration, adding reagents so that the ferrum settles. In addition, you will need a reverse osmosis membrane. When the composition of the liquid is close to the standards, a small set of equipment is sufficient. If you plan to purchase a deferrizer, please contact. Call to get advice and purchase equipment at a competitive price.