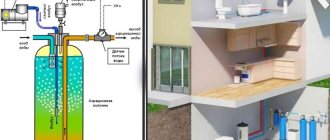
In individual water supply, the main method of obtaining water is lifting it from wells of various depths, and it is often necessary to solve problems associated with its contamination by metal oxides. Iron is most often found in water, which has a negative impact on both health and plumbing and water heating equipment in the house, so deironization of water from a well is an urgent task for many homeowners of autonomous water supply systems.
Well water can have various iron impurities and their concentrations; the type of water treatment technologies used depends on its composition. A consumer who encounters contaminated water is required to have it chemically analyzed in a laboratory, and then, based on the data obtained, decide how to remove iron from the well water.
Rice. 1 Household equipment for iron removal
Norms of iron in water and its varieties
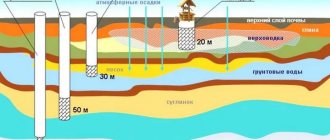
To organize an autonomous water supply for houses, domestic wells are used, the depth of which can reach 200 m. The casing of deep (artesian) springs reaches the calcareous rock and rests on it, the water raised up has a crystal clear appearance without impurities of sand and clay.
However, in most cases, artesian wells, due to the high pressure of the earth layers on the water basins from which water is drawn, produce water with high mineralization. It may contain hydrogen sulfide, oxides of potassium, manganese and iron. The last element is found more often than others in high concentrations and has the most negative effect on the technical characteristics and environmental purity of water.
Sometimes iron is found in shallower well sources in sand or in well water, but in most such cases its percentage is significantly lower than in artesian wells, and water purification methods can be completely different.
The standards established by sanitary services do not allow the use of water with an iron content of more than 0.3 mg per liter for drinking purposes. If this standard is exceeded, it is necessary to purify water from iron using one of several technologies, depending on the concentration and chemical formula of the metal.
When drawing domestic water from artesian wells drilled at a dacha or the territory of an individual cottage, the consumer may encounter the following forms of iron in the water:
Bivalent. Free divalent iron Fe2+ is completely water-soluble, so its presence cannot be determined visually; the criterion may be the smell and taste of water. After settling, soluble Fe2+, as a result of a chemical reaction with oxygen contained in the atmospheric air, is converted into insoluble ferric oxide Fe3+.
When treating water, the solubility of divalent Fe2+ is taken into account and such water is not purified by mechanical methods. A number of technologies for purifying water from iron from a well consist of intensive saturation of water masses with oxygen until the metal is converted into a soluble sediment and then further filtered.
Rice. 2 Appearance of water with iron
Trivalent. As mentioned above, ferric iron Fe3+ is formed after the oxidation of divalent iron, it gives the water a reddish color and leaves a residue on plumbing equipment and dishes. Usually it is possible to get rid of the red color using flow-through carbon filters.
There are other forms of iron present in water in the form of suspensions: bicarbonate Fe(HCO3)2, carbonate FeCO3, iron sulfide FeS and iron sulfate FeSO4, however, these compounds are not often found in artesian waters, have a low concentration and are filtered out by any water treatment method.
Visually, by smell and taste, it is impossible to determine the presence of the listed reagents in water due to their low percentage; the required data is obtained in the laboratory after chemical analysis of the sample taken.
Rust Fe(OH)3. Well-known rust is formed as a result of the interaction of water with iron contained in alloys (steel) in the open air; it consists of ferric oxide Fe2O3 and metahydroxide Fe(OH)3. Since the compounds in rust are water-insoluble, it is easily separated by mechanical filters as a result of water treatment.
Colloidal. Colloidal iron of organic origin is found in water in the form of very small suspended particles no larger than 0.1 microns in size; it cannot be settled and removed by household carbon water treatment filters. It is possible to purify the aquatic environment from such small colloidal fractions only by using a reverse osmosis unit.
Bacterial. These compounds in the aquatic environment are associated with the presence of colonies of bacteria that, in the process of their vital activity, convert the divalent insoluble form of Fe2+ into a trivalent one. Bacteria form a dense iridescent film on the water surface, impart viscosity to the structure of the water, and make it unsuitable for drinking due to an unpleasant odor and bad taste. As in the case of Fe3+, water purification from insoluble bacterial iron can be carried out using household mechanical filters.
Rice. 3 Consequences of using water with high iron concentrations
Reagent cleaning
Another popular option for deferrizing water in a well is the use of reagents. The method is simple and accessible. Therefore, it will not be difficult to carry out all the manipulations yourself. The use of reagents allows you to purify water not only from iron, but also from chlorine and potassium permanganate.
Biological method of water purification is the safest
Harm from iron in well water
To understand why filters are needed to remove iron from well water, you should consider the negative factors of the presence of iron in the well source. If there is a lot of iron in the water, when using it at home:
- The taste of water decreases, it acquires an unpleasant ferrous taste and smell.
- Washing becomes difficult and its quality deteriorates - a high concentration of iron makes the water hard and prevents detergents from working effectively (they do not produce foam), and white laundry takes on a yellow tint.
- Harmful to human health. With regular consumption of water with high concentrations of iron, kidney and liver function is disrupted in the body, pigmentation appears on the skin, teeth turn yellow, hair breaks, the cardiovascular system suffers - this leads to general weakness.
- Hard water with a high iron content is difficult to use for high-quality water procedures; as a result, a person’s personal hygiene suffers and this can affect his health.
- Insoluble Fe3+ causes a noticeable impact on all pipelines for transporting cold and hot water, water consuming and water heating equipment for a summer house and country cottage. Sludge forms in pipes and devices, making it difficult to transport the working fluid.
- The heating elements of washing machines, dishwashers, irons, coffee makers, and kettles are covered with a hard, insoluble oxide crust, leading to a decrease in their operating efficiency, overheating, and rapid failure.
- The narrow input channels of automatic well pumps, plumbing fixtures and fittings become clogged with sludge, leading to blockages and reducing the efficiency of cold and hot water supply systems. The pressure in the line decreases, the volume of water supply decreases, and the operation of the automation in the piping of the water intake submersible well electric pump or pumping station is disrupted.
- A hard-to-remove yellow coating forms on the surface of plumbing fixtures, toilets, bathtubs, sinks, and sinks, reducing the aesthetic appearance of the plumbing fixtures.
Rice. 4 The effect of increased iron content on human health
Membrane method
The membrane method involves the use of microfiltration membranes that retain iron particles. The use of innovative membrane filters in some cases can purify water from iron up to 98%. But unfortunately, this method has many more disadvantages than advantages:
- Rapid clogging of filters with iron particles.
- Few people want to drink distilled water.
This method is used mainly in pharmacology, where clean water is a prerequisite for the manufacture of medicines.
Deferrization of water from a well using reagent-free methods
When implementing reagent-free deferrization, the main consumable component is electricity that powers the working equipment.
It should be noted that the name reagent-free does not fully correspond to reality; in order to finally remove insoluble Fe2O3 precipitate and Fe2+ residues from water, special catalytic water treatment columns (which accelerate the oxidation process and increase filtration efficiency) columns with backfills and carbon filters are used.
The installations are environmentally friendly, do not deteriorate the chemical composition of the aquatic environment, most of them are easy to operate and maintain.
Advocacy
The easiest way to deferrize water from a well with your own hands without significant financial costs for equipment and electricity is to place one or several large containers on the street and settle the water masses in them. In order for settling to occur with the best efficiency, the area of contact between water and air should be as large as possible - this requires the use of large-volume containers.
It is clear that this technology cannot be used in winter; the procedure for obtaining insoluble sediment takes a long time and requires a container with a large surface area, which is difficult to place in the house and impractical to place outside.
The efficiency of the settling system can be increased by supplying water to the tank not directly, but by spraying it to a fine state in the form of water mist.
Rice. 6 Operating principle and structural design of filter aeration and iron removal columns
Compressor aeration
The operating principle of the simplest aeration installation using a compressor is quite simple: a pipeline with holes is lowered into a container with purified water, through which the compressor pumps air. The aqueous environment interacts with the air flow, is saturated with oxygen, soluble iron becomes trivalent and precipitates. Purified water is transferred by overflow to another container and is pumped out from there or removed from the surface layers to post-treatment filters.
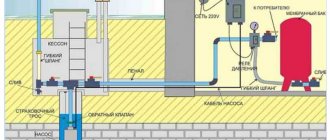
A typical household factory-made compressor deferrization system consists of:
- a compressor that forces air into the container;
- an aeration column in which water masses are saturated with oxygen;
- deferrization columns with a mechanical filter, where additional oxidation and separation of insoluble precipitate occur;
- carbon filter for final purification, screening out residual suspensions of insoluble iron oxides.
- an automatic control unit that periodically pumps insoluble sludge from the deferrization column into the sewer system.
It should be noted that the installation of aeration systems is most simple and less expensive when creating iron removal installations with your own hands.
Fig. 7 Schemes of installations with compressor cleaning
Ejector aeration
The method is effective when the concentration of iron in water is no more than 2 mg per liter; the main components of the ejector installation are an air intake unit and a deferrization column.
The system operates on the following principle: water and atmospheric air under pressure enter into the mixing unit with an ejector located outside or in the automation unit on the deferrization column. In the ejector, the water flow is dissipated and mixed with air masses - as a result, fine water suspensions are saturated with oxygen at a higher intensity than during aeration.
Next, the deferrization of water from the well occurs according to the above technology - purified water with insoluble sediment is sent to the deferrization column and then to a carbon filter for further purification. The liquid with sludge is periodically drained from the column into the sewer according to a program built into the automation system.
Rice. 8 Principle of operation of the ejector and diagram of the ejector installation
Electrolysis
The principle of water purification using electrolysis plants is the decomposition of impurities contained in water under the influence of electric current into active oxidizing agents - oxygen, hydrogen, OH ion hydroxide, chlorine, ozone. The active chemicals react with the water-soluble ferrous iron, which is converted to ferric iron and then precipitated into sludge.
Electrolysis plants (electrolyzers) are suitable for purifying water with an iron concentration of no more than 3 mg per liter; SNiP 2.04.02-84 recommends the use of electrolysis with a chlorine concentration in the water volume of at least 20 mg/l.
Electrolysis is used in industrial water treatment and is practically not used in everyday life due to the high energy consumption of 0.2 kW/m3, the need to introduce additional substances (chlorine-containing components) into the water composition, which provide active reagents during electrolytic decomposition.
Rice. 9 Operating principle of an industrial installation for flow-through water electrolysis UPEV
Ozonation
Ozonation units make it possible to produce trivalent oxygen O3, which is a much more active oxidizing agent than ordinary divalent O2.
The installations have different designs, their main components are an ozonator that produces ozone, a mixing tank in which ozone reacts, converting ferrous iron in well water into insoluble iron. The purified water masses are further purified in a carbon filter, and the sludge accumulated in the tank is sent to the sewer.
An additional advantage of the ozonation system is disinfection and destruction of bacteria, clarification of water and improvement of its taste, and the production of free oxygen as a result of the reaction.
The disadvantages include the high cost of installations, increased explosion hazard, requiring the use of strict safety measures, and complexity of the design, which necessitates the presence of specialists to maintain and repair the equipment.
Rice. 10 Schemes of ozonation installations
Uses of manganese dioxide
The operating principle of this method is similar to the previous one. The emphasis is on the interaction between manganese dioxide and ferrous iron. The result of their reaction is an insoluble compound that precipitates.
Attention! This method involves the use of a column in which the MnO2 compound plays the role of a filter. After a certain period of time, the membrane is filled with sediment, which must be removed manually.
The undeniable advantages of this method:
- purification from hydrogen sulfide and other compounds;
- deferrization;
- long service life.
Filtration using manganese dioxide is a rather expensive method.
The considerable cost of the filter can be attributed to its disadvantages.
Reagent deferrization
The basis of this category of technologies are reagents that enter into a catalytic, chemical or ion exchange interaction with iron dissolved in water. The sediment resulting from the reactions is usually washed into the sewer by backwashing; automation is responsible for controlling all processes.
Catalytic method
The operation of this technology is based on the principle of accelerated chemical transformation of soluble iron by catalytic reagents, after which it passes into an insoluble state. Typically, the reaction occurs in a factory-made deferrization column; a special fill is poured into it and an automation unit is placed on the inlet opening, which is responsible for drainage (discharge of sediment into the sewer system) of the installation.
Ozonation
Enriching water with ozone is another option for iron removal. The process is not much different from the methods described above. Iron is oxidized to a water-soluble state, and the sediment falls to the bottom. The result is water suitable for domestic use.
Scheme: water ozonation
The advantages of the method are undeniable:
- Instant cleansing.
- The reaction between ozone and divalent iron occurs instantly.
- Oxygen enrichment.
- Neutralization of bacteria.
Despite the positive reviews, some disadvantages are inherent in this method. These include:
- High cost.
- Unsafe installation on your own (without the use of qualified labor).
- Possibility of poisoning by harmful substances due to improper use.
- Ozone leak.
Features of the use of installations for household water deferrization
When solving the problem of what to do if there is a high iron content in the well water, the following factors are taken into account:
- Any water deferrization system for a well is designed and installed on the basis of laboratory chemical analysis; it is important for the user to know the amount of hydrogen sulfide impurities, iron ions, potassium, manganese, and organic reagents contained in the water.
- Based on the data obtained, a water treatment system with iron removal is assembled. At high concentrations of iron, systems with aeration are used; if the water contains a lot of hardness salts, complex backfills are used in columns with ion-exchange resins. For a wide range of contaminants, multi-stage water treatment systems are installed.
- The cost of all household installations for iron removal depends on the availability of automation, the number of columns, the brand of backfill and sorbents, and can reach up to 100,000 rubles. The most expensive price is for ozonation units, which, in addition to water purification, provide disinfection and destroy pathogenic bacteria.
- Since deferrization plants neutralize the oxides of all metals, the resulting soft water is not very suitable for drinking or domestic use and requires mineralization.
Rice. 15 Price for equipment for deferrization of water from a well
To remove iron from water, iron removers are used based on installations with columns into which air is supplied from compressors and catalytic, ion-exchange or reagent compositions are poured inside. Usually, deferrization plants are equipped with imported automation, which ensures their uninterrupted operation and self-cleaning functions. The cost of equipment, automation, and consumables for installations for deferrization of water from a well is quite high and, taking into account energy costs, may be unacceptable for owners with low incomes.
What else can be improved?
I would also like to add an ultraviolet disinfectant . Now, for example, we brush our teeth with drinking water, and we also finally rinse vegetables with clean water. Ultraviolet light would allow this to be done safely with tap water. But so far it’s a pity to spend money on such modernization.
I would also put overflow sensors in both barrels to automate the shutdown of the pumps when full. Now I just wait next to him for about 15 minutes. But my hands don’t get around to it, because this procedure is not particularly annoying. Usually, while the water is being collected, I do something nearby in the utility room.